Venous thromboembolism (VTE) is a bad thing. Blood clots of the extremities (i.e., deep vein thrombosis [DVT]) and lungs (i.e., pulmonary embolism [PE]) herald bad outcomes. Both symptomatic and asymptomatic events are associated with escalated risk of mortality. Surveillance studies have shown VTE is a common, possibly preventable, complication during hospitalization. By 2006, three major clinical trials (MEDENOX, PREVENT, ARTEMIS) had demonstrated the benefit of fixed dose low molecular weight heparin (LMWH) or fondaparinux to prevent VTE in hospitalized, acutely ill medical patients. The Food and Drug Administration (FDA) granted this indication to LMWHs, which conveniently dovetailed with the U.S. Surgeon General’s Call to Action to Prevent VTE in 2008 and the ‘National Consensus Standards for the Prevention and Care of Deep Vein Thrombosis’ project between The Joint Commission and the National Quality Forum (NQF). These performance standards fueled a race for hospital staff to find novel ways to identify patients at VTE risk and to deliver parenteral anticoagulants daily. Most hospitals were highly successful categorizing VTE risk on admission, employing validated risk assessment models or developing computer alerts to foster anticoagulant prophylaxis. VTE prophylaxis rates in hospitalized patients (Process Measure VTE-1) and in patients in the intensive care unit (ICU) (Process Measure VTE-2) soared above 90%. The battle to prevent VTE was won!
PERSPECTIVE
VTE-1 and VTE-2 measure whether patients were prescribed any type of VTE prophylaxis (regardless of patients’ risk) and received ≥1 dose of any VTE prophylaxis during the first 2 days of hospitalization, even if they receive nothing after this initial dose. VTE risk exists well beyond 48 hours.
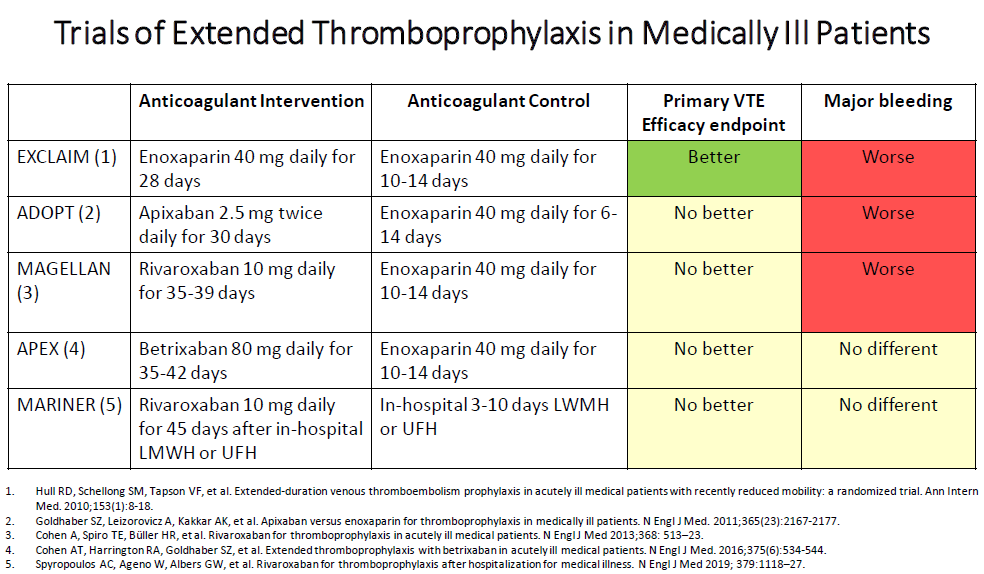
But, over time, more data emerged. Studies suggested that anticoagulants were being over-administered to patients without VTE risk, VTE cases occurred with best care suggesting events may not be preventable, patients were refusing anticoagulant injections, and hospitalization trends promoted early discharge. Academics focused on the early discharge population and showed medically ill patients carried VTE risks home. The battlefield moved- patients were developing VTE in the outpatient setting. Resources were deployed to complete five trials, the anticoagulant armaments now stocked with direct oral anticoagulants (DOACs) (1-5). When the shooting stopped (see Table) and the results analyzed, the efficacy was not convincing and concerns with bleeding risks became amplified. Despite subsequent sub-study analyses identifying benefits in reducing thrombosis (symptomatic VTE, myocardial infarction, non-hemorrhagic stroke, and cardiovascular death) by almost one-third (6,7), FDA approvals for betrixaban (2017) and rivaroxaban (2019), and cost-effectiveness analyses, clinicians have not been compelled to prescribe anticoagulants. Practitioners have cited a low incidence of post-hospitalization VTE, care complications (bleeding, polypharmacy), added work (risk assessment models, care transitions, patient support), the absence of supportive national guidelines, and incremental medication costs (8). The war to prevent VTE has not ended.
PERSPECTIVE
When patients in the MAGELLAN trial with active cancer, dual antiplatelet therapy, gastroduodenal ulcer, pulmonary bronchiectasis/ cavitation/ hemorrhage or recent bleeding episode (<3 months) were excluded, rivaroxaban significantly reduced VTE and VTE-related death over 35 days without increasing major bleeding. Extended VTE DOAC prophylaxis must be tailored for the rare ultra high-VTE Risk, low-bleed risk medically ill patient.
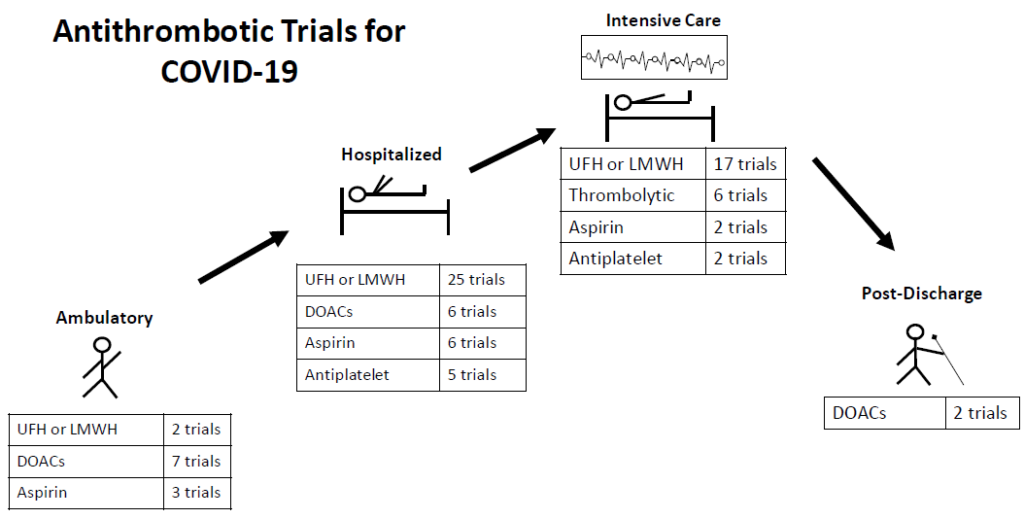
With the COVID-19 pandemic thromboprophylaxis in hospitalized medically ill patients hit the radar again. Early medical reports depicted thrombosis as a common complication, occurring in spite of routine anticoagulant thromboprophylaxis. Experts volunteered treatments ranging from standard-dose prophylactic anticoagulation, to intermediate dose anticoagulation, to therapeutic dose anticoagulation, to aggressive intravenous fibrinolytic administration. Some encouraged DOACs for in-hospital prophylaxis (reducing opportunities for viral transmission and to preserve protective equipment) and extended prophylaxis to prevent thrombotic events. In the recent INSPIRATION trial of ICU patients with COVID-19, intermediate-dose (enoxaparin 1 mg/kg/day) compared with standard-dose (enoxaparin 40 mg/day) prophylaxis did not improve the primary outcome (composite of venous or arterial thrombosis, treatment with extracorporeal membrane oxygenation, or mortality within 30 days) (9). An interim analysis of critically ill patients enrolled in three trials (ACTIV-4a, REMAP-CAP, and ATTACC) comparing therapeutic-dose versus standard prophylactic anticoagulation led to a pause in enrollment because of futility for efficacy and potential excess of bleeding events (10). Shortly after, the same group reported that therapeutic-dose compared to standard anticoagulant prophylaxis in moderately ill COVID patients reduced the need for ventilation or other organ supportive interventions (11). The final reports are eagerly anticipated. Professional organizations (e.g., ACC, ASH, CHEST, ISTH, NIH, WHO) are recommending unfractionated heparin (UFH) and LMWH in standard prophylaxis dosing over DOACs (in the event of clinical deterioration and to avoid drug interactions) in both the ICU and non-ICU settings with only ISTH recommending to consider extended thromboprophylaxis. In addition, there are numerous trials ongoing with COVID-19 infected patients with antithrombotic therapies: 11 trials in ambulatory patients, 50 trials in hospitalized non-ICU patients, and 33 trials in the ICU setting (12) (see Figure). The war to prevent VTE is more complicated than ever before.
PERSPECTIVE
Low dose DOACs are being evaluated in the PREVENT-HD trial (rivaroxaban 10 mg daily in outpatients with symptomatic COVID-19 infection) and the ACTIV-4b trial (apixaban 2.5 mg twice daily in hospitalized patients with COVID-19). Therapeutic dose DOACs are being evaluated in the COVID-PREVENT (rivaroxaban 20 mg daily), ACTIV-4b (apixaban 5 mg twice daily) and the HERO-19 (edoxaban 60 mg daily) trials. Watch for these trial results.
PERSPECTIVE
What can the Pharmacist to do for VTE readiness?
- Evaluate current VTE performance (assessing VTE risk, prescribing and delivering appropriate prophylaxis, VTE event reporting) in your hospital rather than depend solely on Joint Commission metrics.
- Ensure an appropriate VTE prevention strategy is employed in high VTE risk patients and omitted in low VTE risk patients.
- Explain to patients the VTE risk and the benefits of painful injections, to prevent missed doses and improve compliance.
- Follow hospitalized medically ill patients and characterize those patients who return to your facility with VTE complications. You may identify your unique patient population that may benefit from extended thromboprophylaxis.
- Follow the news and national guidelines for recommendations on antithrombotic therapy for COVID-19 infections. Implement practices that are consistent with your patient population.
More Information
References
- Goldhaber SZ, Leizorovicz A, Kakkar AK, et al. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med. 2011; 365:2167-77.
- Cohen A, Spiro TE, Büller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013; 368:513–23.
- Cohen AT, Harrington RA, Goldhaber SZ, et al. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. 2016; 375:534-44.
- Spyropoulos AC, Ageno W, Albers GW, et al. Rivaroxaban for thromboprophylaxis after hospitalization for medical illness. N Engl J Med. 2019; 379:1118–27.
- Spyropoulos AC, Ageno GW, Albers GW, et al. Post-discharge prophylaxis with rivaroxaban reduces fatal and major thromboembolic events in medically ill patients. J Am Coll Cardiol. 2020; 75:3140–7.
- Nafee T, Gibson CM, Yee MK, et al. Reduction of cardiovascular mortality and ischemic events in acute medically ill patients. Circulation. 2019; 139:1234–6.
- Hiatt J, Vazquez SR, Witt DM. Provider perceptions of extended venous thromboembolism prophylaxis for hospitalized medically ill patients. Thrombosis Update 2021, https://doi.org/10.1016/j.tru.2021.100034.
- The INSPIRATION Investigators. Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit. JAMA 2021. doi:10.1001/jama.2021.4152.
- NIH ACTIV trial of blood thinners pauses enrollment of critically ill COVID-19 patients. News release. National Institutes of Health. December 22,2020. Accessed April 27, 2021. https://www.nih. gov/news-events/news-releases/nih-activ-trialblood-thinners-pauses-enrollment-critically-illcovid-19-patients.
- Talasaz AH, Sadeghipour P, Kakavand H, eta l. Recent randomized trials of antithrombotic therapy for patients with COVID-19. J Am Coll Cardiol. 2021. Available at: https://reader.elsevier.com/reader/sd/pii/S0735109721004587?token=EFB2A5973FD8269A812DB7858FC413A5029912047E3C353A7A16F1F2C3CB72044EA493F0EFDEC13F88C3527E580124E2&originRegion=us-east-1&originCreation=20210429181729